How do the cells ensure the physical process of chromosome segregation and cell division?
How and why do germ cells stay connected after cytokinesis? How are the intercellular bridges stabilized?
Mechanistic understanding of cell division and architecture.
How do the cells ensure the physical process of chromosome segregation and cell division?
How and why do germ cells stay connected after cytokinesis? How are the intercellular bridges stabilized?
How does the composition of microtubules influence microtubule structures and cell function? How do mutations affect microtubule function?
To address these important questions, we are using a multidisciplinary approach and have fantastic collaborations within and outwith Edinburgh.
To maintain their genomic integrity, eukaryotic cells must replicate their DNA faithfully and distribute it equally to the daughter cells. The segregation of chromosomes is mediated by the microtubules. Microtubules depend on motor proteins to assemble into a spindle and segregate chromosomes. However, we are studying how the activities of individual motors and their interacting regulatory networks generate physiological cellular function such as chromosome segregation.
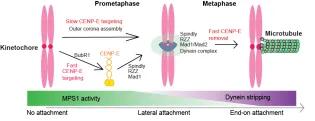
The mitotic CENP-E is a large kinesin motor (312kD) that localizes to unattached kinetochores to align the chromosomes into the equator of the cell, where they biorient. At the metaphase to anaphase transition, CENP-E relocalizes to the central spindle, although its function there is unclear because of its essential role in chromosome alignment.
We have defined the domain of CENP-E, which associates with kinetochore-bound BubR1 during checkpoint activation and showed this recruitment is essential for chromosome alignment (Legal et al, 2020). We recently showed CENP-E is also recruited to the outer corona of kinetochores using structural biology and functional studies (Weber et al, 2024). Our work defines the mechanisms coupling CENP-E to unattached kinetochores preceding biorientation.
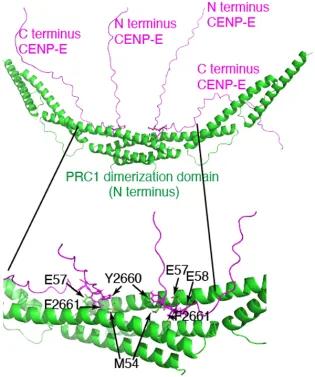
Human CENP-E motors in vitro are fast processive motors, but its C terminus is auto-inhibitory (Craske et al, 2022). It associates with PRC1 through a bipartite C terminal motif both in vivo and in vitro, to target to overlapping microtubules within the spindle midzone in late mitosis. We showed CENP-E slides antiparallel microtubules when in presence of PRC1 in vitro.
Mitotic phosphorylation regulates the timing of the interaction, by reducing the affinity of the human CENP-E:PRC-1 interaction until chromosome biorientation and segregation have taken place, allowing spatial and temporal coupling of CENP-E relocalization and function at overlapping antiparallel microtubules in anaphase (Gluszek-Kustusz et al, 2023). In the absence of mitotic motors recruited to PRC1, the central spindle is disorganized and the midbody, resulting from central spindle compaction, does not assemble, leading the cytokinesis failure.
We seek to understand how microtubule motors coordinate chromosome segregation, and how they organize the microtubules central spindle to build the midbody.
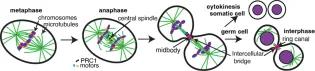
In some types of both male and female germ cells, cytokinesis does not go to completion. Instead the intercellular bridge (IB) are stabilized over days and the midbody matures into a ring structure, called a ring canal. The germ cells form a cyst connected by the IBs, to allow material exchange. Genetic disruption of IBs in germ cells is associated with infertility and subfertility.

In male mammalian germline cells, IBs are critical for spermatogenesis and allow molecule transport to compensate for gene imbalance of haploid genomes and suppress meiotic gene drive. We seek to understand how the IBs are assembled, stabilized and what function they play in mammalian germ cells using molecular and structural approaches, combined with mouse spermatogonial stem cells and tissues (collaborators: D. O’Carroll and I.Adams, University of Edinburgh).
We seek to understand the role of tubulin isotype diversity in the cytoskeletal organization at the molecular level and the molecular basis for isotype-specific associated tubulinopathies at the physiological and organism level. Tubulin is a major anti-cancer drug target. However, an important feature of tubulin is that there are many different isotypes. The isotype-specific response to tubulin-targeting drugs is not known. We are collaborating with Prof Alison Hulme (School of Chemistry) to design isotype-specific tubulin drugs and develop cellular assays to develop these drugs.

This article was published on