Our lab is interested in the organisation, establishment and maintenance of specialised chromatin states. Epigenetic transmission of centromere identity through many cell generations is required for proper centromere function and when perturbed can lead to genome instability and disease. We use Drosophila and human tissue culture cells as model organisms to address the following questions:
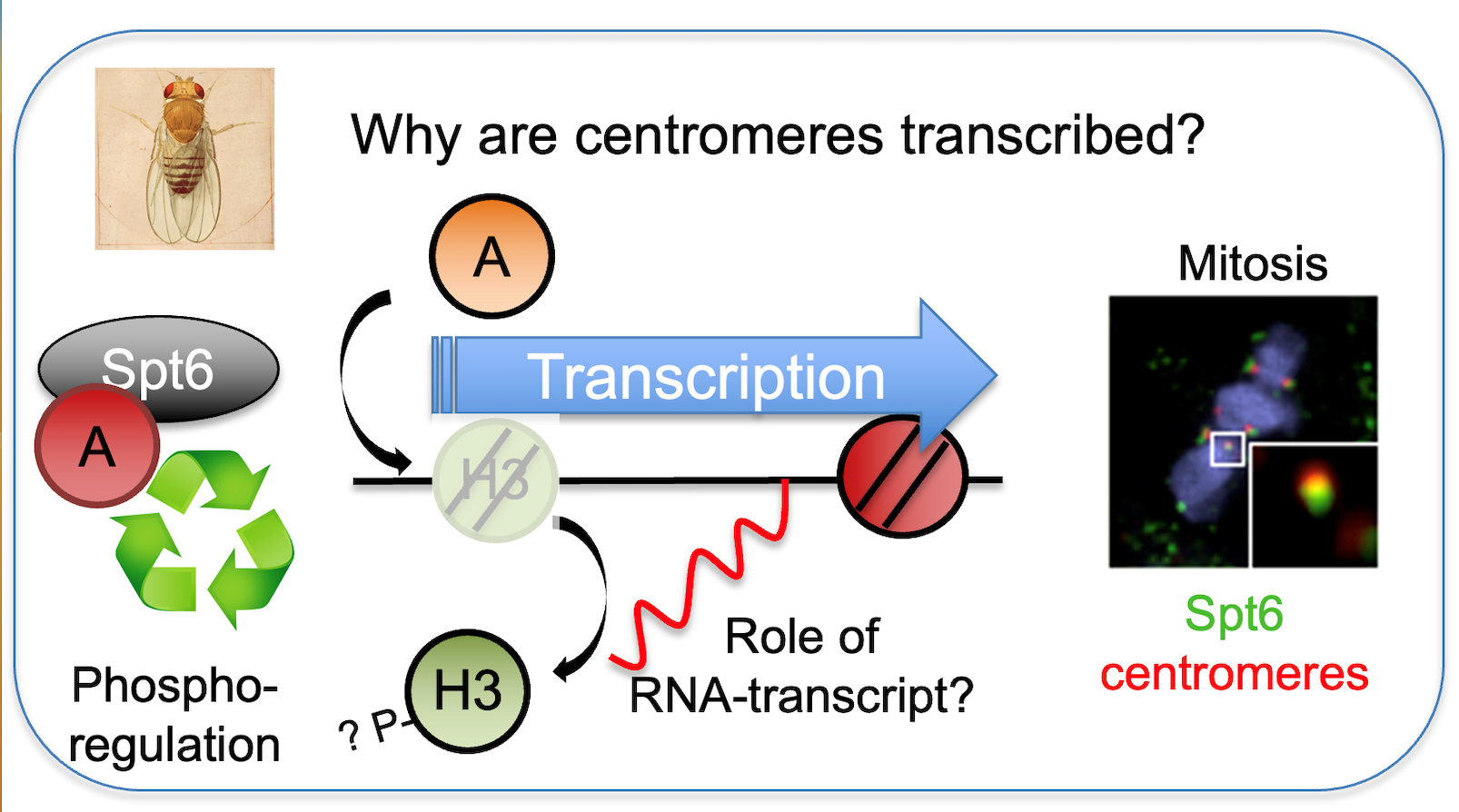
What is the role of transcription at the centromere?
Loading of CENP-A at the centromere occurs outside of S-phase and requires the removal of H3 “placeholder" nucleosomes. Transcription at centromeres has been linked to the deposition of new CENP-A, although the molecular mechanism is not understood. Using fast acting transcriptional inhibitors we demonstrate that centromeric transcription is required for loading of new dCENP-A and promoting dCENP-A transition from chromatin association to nucleosome incorporation (Bobkov et al., 2018). Unlike placeholder nucleosomes, previously deposited CENP-A is specifically retained by Spt6 both in human and Drosophila cells, identifying Spt6 as a CENP-A maintenance factor that ensures the stability of epigenetic centromere identity (Figure 1). We are currently investigating the molecular mechanism how some histones like CENP-A are maintained while others like H3.3 placeholders are evicted to preserve epigenetic centromere identity.
How is the centromeric chromatin fiber organised?
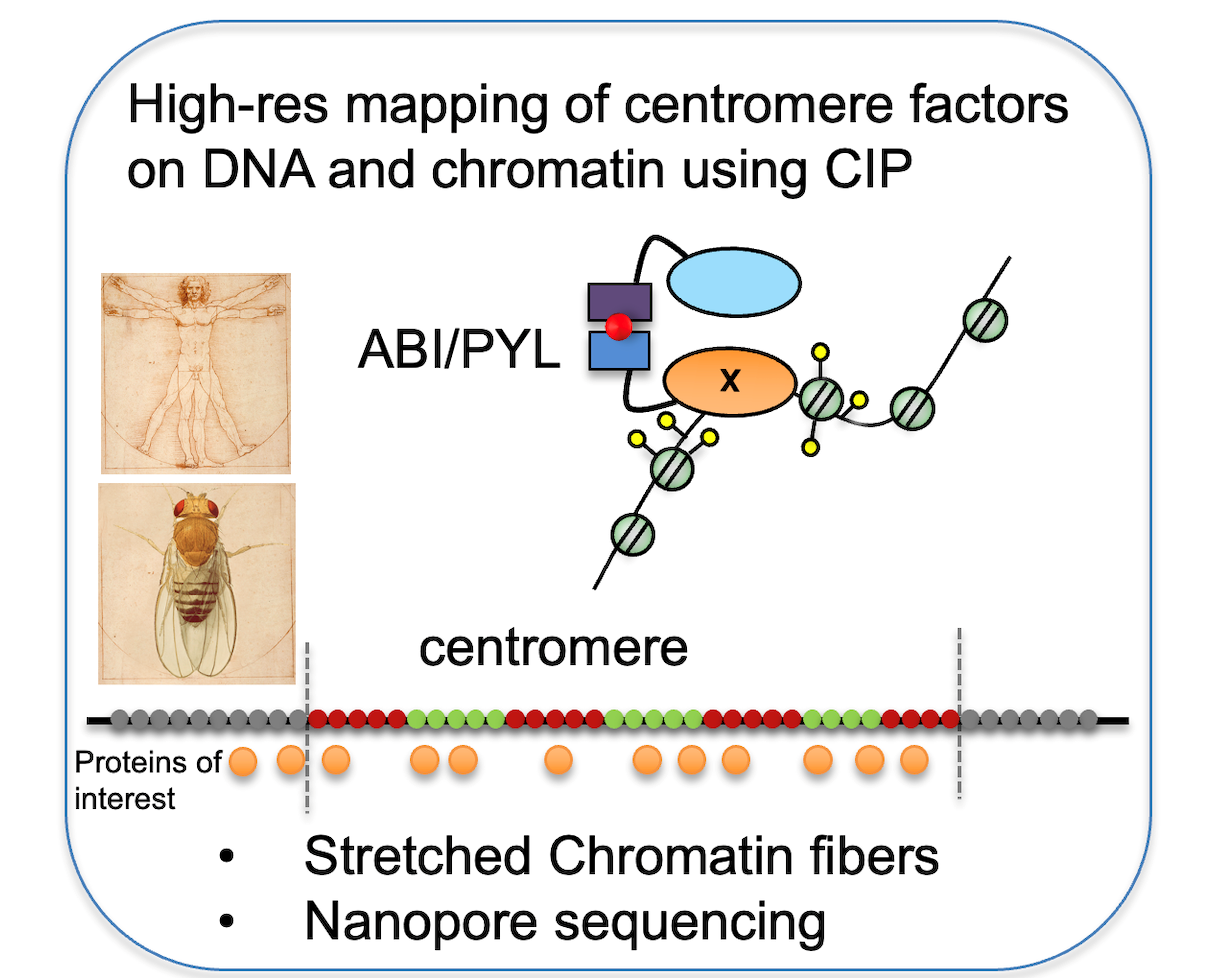
To map centromere proteins on the linear centromeric chromatin fiber, we have recently developed a novel approach where proteins-of-interest fused to ascorbate peroxidase (APEX) or Turbo ID leave a “footprint” on the underlying nucleosomes through proximity-biotinylation. With this methodology we have described novel localization patterns of a subset of centromere proteins at human centromeres (Kyriacou and Heun, 2018). We are extending this approach to proteins localising to all layers of the centromere, including the inner centromere, CENP-A loading factors and the outer kinetochore.
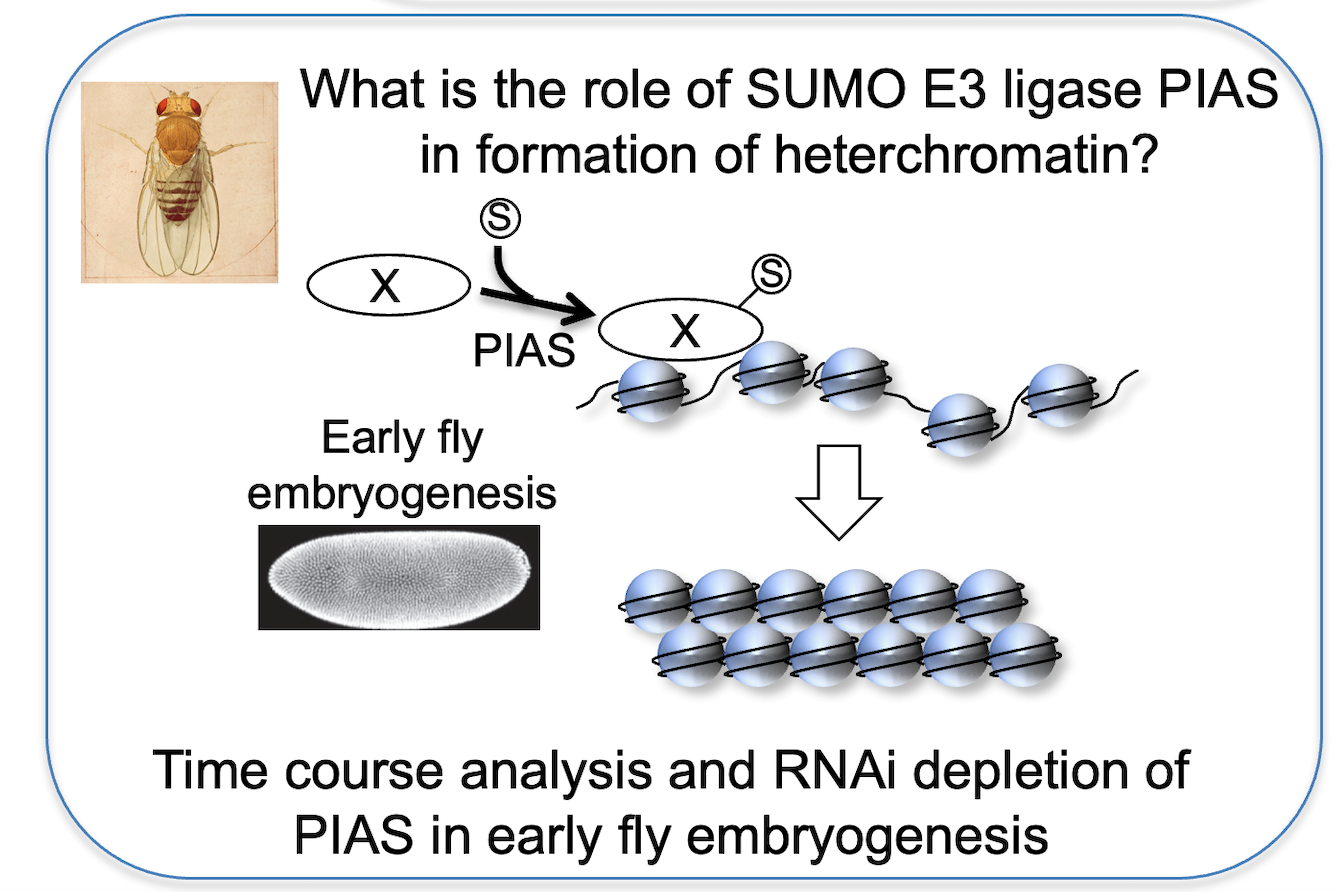
How does Su(var)2-10/PIAS contribute to heterochromatin organisation?
The Su(var)2-10 gene has been originally identified in position-effect-variegation (PEV) assays designed to uncover proteins involved in heterochromatin formation. Cloning of the gene revealed its homology to the protein family SUMO E3-ligase PIAS (Protein Inhibitor of activated STAT), but how sumoylation promotes heterochromatin formation remains unknown. While PIAS does not localise to pericentric heterochromatin in somatic cells, it is enriched next to centromeres in early fly embryogenesis, suggesting a role in heterochromatin establishment. Specific depletion of PIAS as this point of development will shed light on the link between PIAS’ SUMO targets and chromatin organisation.